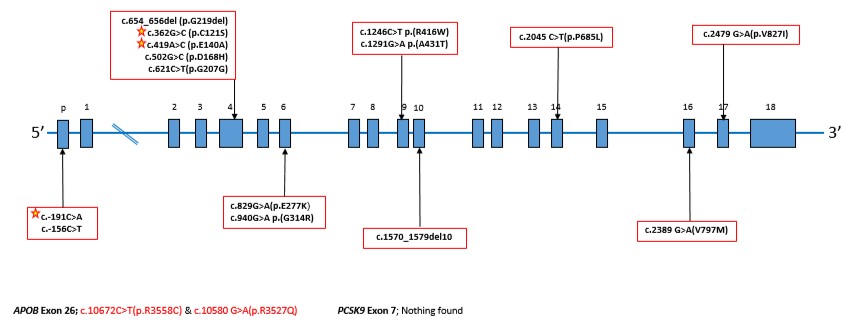
Graph: Diagrammatic representation of the LDL receptor, annotated with DNA sequence changes in the Israeli FH cohort. The stars indicate novel variants seen only in the Israeli population. –
The Center for the Treatment and Prevention of Atherosclerosis at the Hadassah Medical Center’s Heart Institute leads Israel’s effort to identify and treat families with high levels of cholesterol in their blood stream.
Known as Familial Hypercholesterolemia (FH), the condition is a result of one of the most common genetic disorders. Carriers of the FH mutation have a six times higher risk than the general public of developing early onset coronary artery disease and experiencing sudden death. It is estimated that 1 in 300 people has FH. Of patients with premature coronary disease, 1 in 20 has FH. Because this disorder is inherited in an autosomal dominant fashion, one defective gene inherited from either parent is enough to lead to high cholesterol.
The FH mutation translates into a defect in the body’s ability to clear low-density lipoprotein cholesterol (LDL-C)—the “bad” cholesterol. Most commonly, the defect is in the LDL-C receptor of the liver cells. Normally, this receptor attaches to the LDL-C particles in the blood, enabling the body to excrete these particles through the liver into the bile. In cells with defective LDL receptors, this normal process is inefficient, resulting in high plasma levels of LDL-C. The LDL then accumulates in blood vessels and causes atherosclerotic disease (narrowing of the arteries due to a buildup of plaque).
Because FH is associated with significant risk, it is important to make the diagnosis in a timely fashion and initiate treatment.
Recently, a research team at Hadassah’s Heart Institute tested families suspected of being FH carriers, using the newest DNA sequencing technologies. The researchers identified four previously unknown mutations–three in the Jewish population and one in a large Druze family. Results of this analysis were published in the prestigious ” medical journal, Atherosclerosis, in 2017.
Understanding FH mutations has changed considerably over the past 20 years, both because of immigration patterns and access to newer technologies to identify mutations. Researchers identify what is called a “Founder Effect,” which occurs when a group becomes separated from the general population, and remains isolated. This can lead to a high incidence of what are normally unusual diseases in that population, as the genetic mutations of the “founders” of the population spread into the next generations. Founder effects have been identified in many populations in Israel. In the Ashkenazi Jewish population, founder effects date back to the 14th century. Two decades ago, the Hadassah lipid team reported a series of genes that were responsible for a large percentage of the FH in Israel. They screened 192 families and found 15 different mutations. The FH patients harboring these 15 mutations originated from 10 countries (Hungary, Iraq, Israel, Lebanon, Lithuania, Morocco, Poland, Romania, Russia, and Syria). .
The Hadassah laboratories have been, and continue to be, leaders in this field, as its researchers work on new strategies to improve early screening. In addition, plans are underway to further explore these new mutations to see if any of them provide clues as to how to prevent and treat atherosclerotic disease.
