Data from the first patient cohort of the Phase I/IIa Hadassah Medical Organization clinical trial with the stem cell product, OpRegen®, aimed at slowing the progression of the advanced form of dry age-related macular degeneration (dry-AMD), will be presented at the International Symposium on Ocular Pharmacology and Therapeutics (ISOPT) on Friday, December 2, 2016, in Rome, Italy.
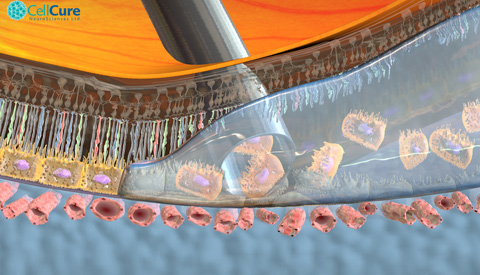
OpRegen®, comprised of retinal pigment epithelial (RPE) cells derived from human embryonic stem cells, was developed by BioTime Inc’s subsidiary, Cell Cure Neurosciences Ltd., together with Key Developers Prof. Eyal Banin, Director of Hadassah’s Center for Retinal and Macular Degenerations and a leading investigator for this trial, and Prof. Benjamin Reubinoff, head of Hadassah’s Human Embryonic Stem Cell Research Center and Chief Scientific Officer of Cell Cure. “The cells were created without the use of animal-derived products through a proprietary directed differentiation process to produce a highly purified population of RPE cells,” explains Prof. Reubinoff.
Prof. Banin notes that OpRegen has been “administered successfully in the first cohort of patients, with no serious adverse events.” In addition, retinal imaging suggests that OpRegen RPE cells are able to engraft.
The study involves the evaluation of the safety of three different dose regiments of OpRegen. The first Phase I/IIa patient cohort received an initial targeted dose of 50,000 cells. The Data Safety Monitoring Board (DSMB), an independent group of physicians and medical experts which is closely monitoring the clinical trial, has thoroughly assessed the safety profile of the first cohort of subjects and given its recommendation that the company continue the trial with the second cohort at a higher dose of 200,000 cells.
Enrollment in the second cohort is expected to be completed by the end of 2016. Depending on the outcome of the DSMB’s review of the second patient cohort, approval to begin administering the 500,000 cell dosage to the third patient cohort could be provided early next year. In addition, BioTime reports, the first OpRegen United States clinical trial site is expected to be selected in the near future.
“We are looking forward to the opportunity to present data from the first patient cohort at ISOPT,” said Adi Mohanty, Co-Chief Executive Officer of BioTime. “OpRegen’s progress in the clinic is gaining momentum and we have already treated patients with 200,000 cell doses. It is in these higher cell dose cohorts where we believe OpRegen has the potential to demonstrate more meaningful clinical outcomes and we expect to start reporting on these data in early 2017. Our goal is to develop a treatment that can serve the millions of dry-AMD patients for whom there are currently no FDA-approved therapies.”
OpRegen had received “Fast Track” designation from the Food and Drug Administration for treatment of the advanced form of dry-AMD. Details of the trial and about a patient’s eligibility are available at https://clinicaltrials.gov/ with the following Identifier: NCT02286089 (dry-AMD).
