Collaborating with researchers at the Weizmann Institute of Science, Dr. Marc Gotkine, head of the Hadassah Medical Center’s ALS and Motor Neuron Disease Clinic, along with Michal Zabari of Hadassah’s Department of Neurology, have shown in a preclinical study in mice that intestinal microbes, collectively termed the “gut microbiome,” may affect the course of amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease.
In fact, one strain, in particular, may slow the disease’s progression.
The collaboration with Weizmann began in 2014, according to Dr. Gotkine. Initially, through a series of experiments, the researchers demonstrated that ALS-like disease symptoms in mice worsened after these mice were given broad-spectrum antibiotics to wipe out a substantial portion of their microbiome. These findings indicated the possibility of a link between gut microbial changes and accelerated ALS development in mice that were genetically engineered to be prone to ALS.
In a further study, the researchers identified 11 microbial strains that became altered in ALS-prone mice as the disease progressed or even before the mice developed overt ALS symptoms. When the scientists isolated these microbial strains and gave them one by one, in the form of probiotic-like supplements, to ALS-prone mice following antibiotic treatment, some of these strains had a clear negative impact on the ALS-like disease. But one strain, Akkermansia muciniphila, significantly slowed disease progression in the mice and prolonged their survival.
When the researchers subsequently examined the microbiome and metabolite profiles of 37 human ALS patients and compared them to those of family members sharing the same household, the gut microbiomes of the ALS patients proved distinctly different in composition and functional features. Many microbial genes were significantly suppressed in ALS patients.
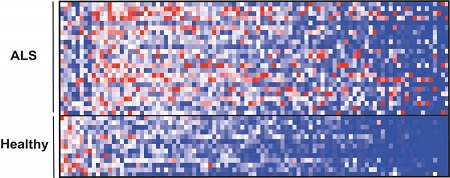
An analysis of thousands of small molecules in the blood also revealed a distinct pattern in ALS patients when compared with the pattern in a control group. Moreover, the researchers report, there was a correlation between reduced levels of certain molecules and the degree of muscle weakness in ALS patients.
“The results of the study,” says Dr. Gotkine, “have shown us that in ALS patients, we see findings similar to those we have seen in mice, which gives great hope that the intervention that caused the disease in mice may also be relevant in humans.”
Dr. Gotkine emphasizes, “it is important to note that human research is long and complicated. ALS patients are patients whose mobility is very low and arriving at the Hadassah clinic at any time to be part of the research process requires tremendous mobilization of the patient and his family. With the help and assistance of the Hadassah research coordinator, Michal Sabri, we were able to do that. “
Providing a historical perspective on the advances in ALS research and Hadassah’s contribution, Dr. Gotkine notes also that the infrastructure for the current research on ALS was established in 2009. “At that time,” he says, “the Israeli ALS Association approached me with the idea of establishing a new clinic for ALS patients. From the day the clinic was set up, we started collecting demographic and clinical data on the patients and entering them into the database we created. Today, Hadassah has the world’s most detailed and largest database of ALS patients.”
See the latest study highlighted in Nature.
Read more in the Hebrew article, Kol Hair.
Caption and Photo Credit
The repertoire of small molecules (metabolites) in blood, many of which originate in the microbiome, showed a different pattern in patients with ALS (top) compared with healthy individuals (bottom).
Photo Credit: Weizmann Institute of Science
